Project Approach
The present project involves the initial development and testing of an acute stroke treatment two man mobile hyperbaric oxygen chamber equipped ambulance which published scientific clinical trial data indicate will “stop the clock” on the required FDA 3 hour post stroke administration window of the thrombolytic tPA (the only FDA approved stroke treatment drug). This will extend the service area an ambulance can travel to bring acute stroke patients to a major medical center stroke center. This will be a major public health advance extending the FDA approved stroke treatment drug tPA administration to thousands more stroke patients each year, as well as better treatment for other kinds of acute stroke.
In collaboration with American Emergency Vehicle Company, the largest ambulance manufacturing company in the U.S., we are developing a prototype hyperbaric oxygen chamber-equipped stroke treatment ambulance.
Once we complete the design, building and safety testing of this prototype ambulance, the ambulance will become the foundation for a Phase II application to the National Institutes of Health to perform human clinical trial testing that’s necessary for FDA approval of the scientific principals outlined in our 2011 publication of successful hyperbaric oxygen human stroke treatment by McCormick, Houle, Saltzman, Whaley and Roy. Our work is directed towards using ambulance-based hyperbaric oxygen as a bridge to better acute stroke treatment with tissue plasminogen activator (tPA) for thousands more stroke patients each year.
Donation of Hyperbaric Oxygen Chamber
Dr. James McCormick received a letter from Dr. Robert C. Whaley, head of the U.S. Navy Diving Department, documenting the provision of the hyperbaric oxygen chamber to Wake Forest School of Medicine to be used in the design, development and testing of an ambulance to safely transport stroke patients under pressure.
 Photo: James McCormick, PhD with the donated hyperbaric chamber from the U.S. Navy Diving department.
Photo: James McCormick, PhD with the donated hyperbaric chamber from the U.S. Navy Diving department.
Project Overview
- Year 1 – We will complete the manufacture of the custom-built ambulance box, including all plumbing and mounting necessary to run our two-man mobile U.S. Navy pressure chamber in the ambulance box.
- Year 2 – We will complete the mating of the ambulance box to the ambulance chassis, and we will carry out pressure testing of the hyperbaric oxygen chamber in the ambulance—first in a static situation and second in road tests. No human subjects will be used in this study. Chamber and ambulance safety testing will be accomplished with engineering studies.
- Years 3 through 5 – We plan to start clinical trials with acute stroke patients.
- Stroke ambulance comes to the patient’s home when they call 911 and is immediately used to reverse the patient’s stroke conditions in the hyperbaric oxygen chamber on the ambulance after an NIH stroke scale clinical exam is given.
- Hyperbaric oxygen chamber treatment of the patient is continued in the ambulance on the way to a hospital-based stroke center.
- Arriving at the hospital, patient is removed from the pressure chamber on the ambulance and taken into the emergency room for a computerized tomography (CT) scan after an NIH stroke scale clinical exam is given.
- The CT scan in the emergency room determines if the patient is having a clot-based ischemic stroke (85% of cases) requiring transition to tPA thrombolytic therapy. If the CT scan determines the patient is having a hemorrhagic stroke (15% of cases) the patient is triaged to alternate medical and or surgical care.
Innovation
Hyperbaric oxygen treatment will be a bridge to tPA therapy, with hyperbaric oxygen having the ability to stop the FDA three-hour post-stroke time window for safe tPA administration by preserving brain tissue in a safe hyperbaric oxygen stasis. This will extend the service area an ambulance can travel to bring acute stroke patients to a major medical center stroke center, thus opening up this FDA-approved stroke treatment drug to thousands more patients each year.
This will be a major public health advance extending the FDA-approved stroke treatment drug tPA administration to thousands more stroke patients each year.
Project Goals
- Design – Designing and building a ground ambulance equipped with a two-man hyperbaric oxygen chamber, that published scientific clinical trial data indicate will “stop the clock” on the required FDA three-hour post stroke administration window of the thrombolytic tPA (the only FDA approved stroke treatment drug).
- Pick up – When the acute stroke patient is picked up at their home, the FDA three-hour tPA time clock can be stopped.
- Range – Increase the range an ambulance can go to get the acute stroke patient to the major medical center stroke center for tPA.
Presently, with the time needed to work up a patient for tPA within the three-hour window, an ambulance can only travel about 15 minutes or less with the acute stroke patient.
Our published data in the literature (McCormick, Houle, Saltzman, Whaley and Roy, 2011) suggests that this hyperbaric oxygen treatment in route to the stroke center will be synergistic with tPA increasing efficacy and safety. The hyperbaric chamber is being engineered into a prototype ambulance and safety certified and tested. This is in preparation for a Phase II Clinical Trial on Acute Stroke Patients.
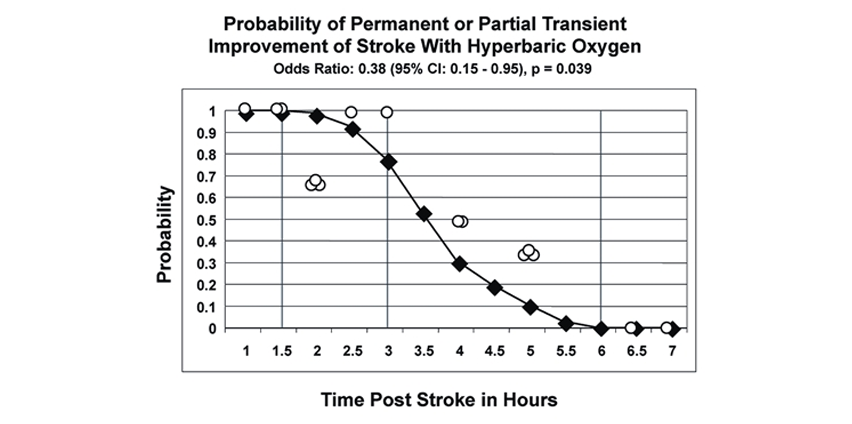
Hyperbaric Oxygen Revival of Acute Stroke Patients. Excellent to 90 Minutes Post Stroke (100%), Good to 3 Hours Post Stroke (78%).
Patient Safety and Design
The hyperbaric oxygen acute stroke treatment ambulance is a groundbreaking venture endorsed by the Undersea and Hyperbaric Medical Society. . Dr. Robert Whaley, Past Director of the U.S. Navy Diving Department, and Principle Investigator Dr. McCormick, will be leading our ambulance construction patient safety certification.
Dr. Robert Whaley, past Director of the U.S. Navy’s Diving Division, was responsible for the development, production and fleet implementation of the U.S. Navy’s shipboard and portable hyperbaric systems and equipment. Now President and CEO of his own Diving/Pressure Chamber consulting company, DynaMech Analysis LLC, Dr. Whaley will collaborate with Dr. McCormick on this project as a consultant on operational, safety, and engineering issues.
Dr. Roy Alson, as Medical Director of the Forsyth County Emergency Medical Service; Medical Advisor for Disaster Services, NC Office of EMS; Assistant Medical Director of the North Carolina Office of Emergency Medicine; Chairman of the Disaster Preparedness and Response Committee at ACEP; and Professor of Emergency Medicine at Wake Forest Medical School, will also add his experience to designs for Patient Safety.
Our collaborator, the American Emergency Vehicle (AEV) ambulance manufacturing company, is a worldwide leader in ambulance safety design and highway regulation compliance. AEV has over a 25-year working relationship with the Wake Forest Baptist Medical Center designing and building Air Care and Brenner’s Children Ambulances.
