The Peters Lab investigates the mechanisms through which surgery, nerve injury or arthritis produce changes in the peripheral nerve, spinal cord and brain that lead to the transition from an acute to chronic pain state.
Research Projects
Role of stress-Induced locus coeruleus dysfunction on pain resolution and disability after surgery
The failure of pain to resolve after surgery has a significant impact on the physical function, emotional well-being, and overall quality of life for those affected that often leads to long term disability. As a result, the identification of interventions to prevent or more effectively treat chronic pain after surgery (CPAS) has the potential to significantly impact public health.

Clinically, a person’s capacity to engage endogenous analgesic circuits has been shown to predict a higher incidence of pain and disability for several months after surgery. The causal mechanisms responsible for this relationship is not completely understood; however, chronic stress and persistent pain both profoundly alter monoaminergic pathways. We have previously shown that descending spinal noradrenergic circuits that originate in the locus coeruleus (LC) are important for resolution of mechanical hypersensitivity following incision and partial nerve injury. We have also demonstrated that exposure of rodents to repeat psychosocial stress prior to surgery significantly slows resolution of postsurgical mechanical hypersensitivity. We propose that increased tonic LC activity induced by chronic stress disrupts noradrenergic circuits and receptor- mediated signaling pathways resulting in impaired recovery across multiple behavioral domains (sensory, affective and cognitive).
In the lab, we are using a variety of techniques to examine the relationship between patterns of LC activity and resolution of postsurgical recovery including in vivo LC electrophysiology in combination with behavioral and viral vector mediated optogenetic/chemogenetic approaches. Recognizing emerging evidence that the LC is a functionally heterogeneous structure, we are selectively exciting/silencing discrete LC projections (spinal cord, amygdala, prefrontal and cingulate cortex) to better understand the contribution of specific projections and terminal noradrenergic signaling to cognitive dysfunction, negative affect and pain related behaviors in acute and persistent postsurgical pain models. Ultimately, we hope to use this information to identify therapeutic strategies to improve postoperative outcomes in the clinic.
This work is being conducted in collaboration with Jeff Martin, PhD, (Anesthesiology) and James Eisenach, MD, (Anesthesiology) and is funded by NIGMs P01GM113852 (Eisenach).
Dissecting neural circuitry involved in driving musculoskeletal pain.
Osteoarthritis (OA) and inflammatory joint pain affect over 50 million adults in the United States, and both are leading causes of chronic pain, disability and reduced quality of life. Our knowledge of the distribution, phenotype and factors that drive plasticity of sensory neurons that innervate the bone and joint are incomplete.
It has been established that bone and joint are innervated by a discrete population of sensory neurons distinct from sensory neurons that innervate skin including predominantly small (C fiber) unmyelinated peptidergic afferents (SP+, CGRP+ NF200-) and medium (Aδ fiber) myelinated (CGRP+ NF200+) afferents. Studies have shown that mouse bone and knee joints are minimally innervated by small (C fiber) unmyelinated non-peptidergic (IB4+ P2X3+) sensory neurons. Notably, the majority of sensory neurons that innervate the bone contain TrkA the endogenous receptor for nerve growth factor (NGF).
Distinct roles for subsets of sensory neurons have been proposed in nociceptive processing and may drive unique pain symptoms. It is increasingly being recognized that ongoing pain associated with musculoskeletal pain conditions may be mechanistically distinct from movement evoked pain. We have identified a subpopulation of high threshold mechanosensitive afferents that densely innervate bone and joint tissue. We hypothesize that sensitization of this population of afferents from injury or inflammation of the joint are significant contributors to movement evoked pain and the transition from acute to persistent joint pain. Thus, pharmacological strategies that target these afferents may have the potential for reducing pain associated with musculoskeletal diseases.
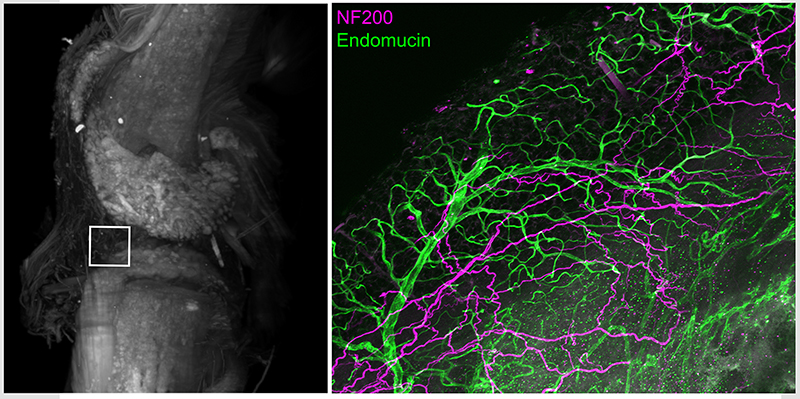
Intact whole mouse knee joint imaged using light sheet microscopy displaying autofluorescence (white) to reveal structural details. High power confocal image of infrapatellar fat pad region in knee joint following tissue clearing and immunohistochemistry with antibodies for vascular endothelial cells (endomucin, green) and myelinated sensory neurons (NF200, purple)
As part of this proposal, we will conduct anatomical studies to better understand the distribution of several molecularly defined subsets of sensory neurons in bone and joints using deep tissue clearing and 3D light sheet and confocal imaging (Figure above). We are also developing several approaches to modulate discrete neuronal subsets to examine their functional contribution to arthritic joint pain. We use a battery of behavioral assays for ongoing and movement related pain in combination with optogenetic/chemogenetic modulation of sensory neuron subsets in several transgenic mice. We seek to combine these methods with molecular profiling of afferents that innervate bone/joint using single cell RNA sequencing and in vitro and /or in vivo physiological assessment of subsets of sensory neurons under normal and arthritic conditions.
This work is being conducted in collaboration with Juan Miguel Jimenez-Andrade, PhD, (Univ. of Tamaulipas, Mexico) and Jeffrey Willey, PhD, (Radiation Oncology) and is funded by NIAMs R21 AR078366 (Peters)
Molecular cross talk bone metastatic prostate cancer and nociceptive neurons
Bone pain is a particularly problematic consequence of skeletal metastasis in prostate, breast and lung cancer. We are conducting studies to better understand the tumor, immune cell and sensory neuron interactions that occur within the bone microenvironment responsible for driving bone cancer pain.
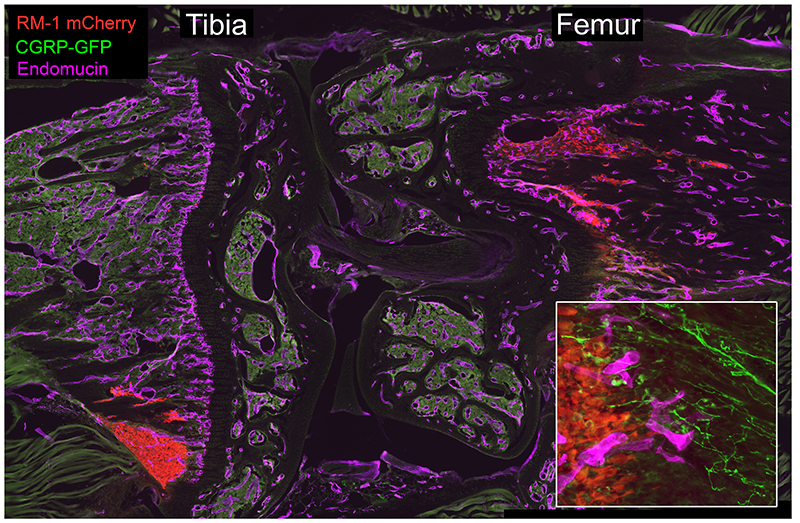
RM1-mCherry prostate cancer cells (red) home to the metaphysis of the tibia and femur following intracardiac injection in C57Bl6 mice. Sensory neurons (CGRP-GFP, green) and endothelial cells (purple) are closely-associated with migrating PCa cells (inset).
For these studies, we have developed and characterized several mouse models of bone cancer pain (prostate, breast, lung) including syngeneic and xenograph models utilizing human cancer cell lines. We employ a battery of behavioral assays to examine the effects of standard and novel analgesics on ongoing or spontaneous pain and movement evoked pain and simultaneously assess their impact on tumor growth and bone remodeling. A secondary goal of these studies is to examine the contribution of sensory neuron subsets in the regulation of the metastatic disease process including dissemination of cancer to bone, tumor growth within bone and tumor-induced pathological bone remodeling.
For these studies, we are using several transgenic mice and viral vector-based approaches to knock down neuropeptides (e.g. CGRP, SP), and associated signaling pathways, in subsets of sensory neurons. We hope this information will help us to develop and identify novel therapeutic agents that can eradicate bone metastatic diseases and relieve associated bone pain.
This work is being conducted in collaboration with Yusuke Shiozawa, MD, PhD, (Cancer Biology) and is funded by DOD W81XWH-17-1-0542 (Peters), DOD W81XWH-17-1-0541 (Shiozawa) and NCI R01CA238888 (Shiozawa).
