Cardio-Oncology Bench to Bedside The goal of our research program is to minimize cardiovascular disease as a consequence of cancer therapies and we seek to bridge basic science with clinical research to advance effective diagnostic tools and interventions. Through murine, nonhuman primate models and clinical studies, we explore the underlying causes of cardiac fibrosis and cardiomyocyte dysfunction induced by cancer therapies, and their contribution to left ventricular dysfunction and heart failure. We frequently employ state-of-the-art cardiac magnetic resonance techniques – similar to those used clinically- to non-invasively evaluate cardiac tissue characteristics across species and enhance the rapid translatability of our findings.

Research Highlights
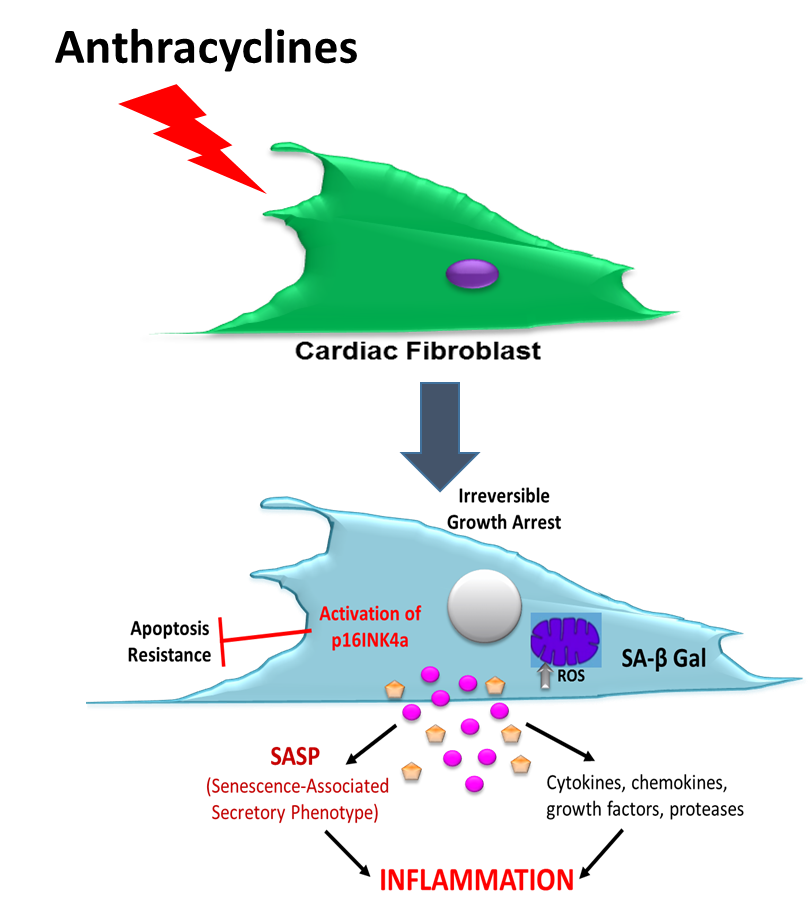
Cellular Senescence: A Novel Mechanism of Doxorubicin-induced Cardiotoxicity
Recently, our group has demonstrated that cardiac fibrosis also contributes to left ventricular (LV) dysfunction and heart failure symptoms following anthracycline-base chemotherapy and now we have evidence that doxorubicin (a widely used Anth-bC) has a direct effect on cardiac fibroblasts to produce excess collagen. This project focuses on a candidate mechanism by which anthracyclines may promote cardiac fibroblast activation and fibrosis: a stress response known as cellular senescence. Cellular senescence is characterized by permanent arrest of cell proliferation, mitochondrial dysfunction, and development of a senescence-associated secretory phenotype (SASP). We have now evidence that cardiac fibroblasts exposed to doxorubicin indeed develop mitochondrial dysfunction and become prematurely senescent.
Accordingly, our specific aims are designed to answer the following questions:
- What are the proteomic constituents of the cardiac fibroblast secretome induced by doxorubicin, and do they stimulate a pro-fibrotic phenotype in previously healthy fibroblasts?
- Is there a causal role for mitochondria dysfunction in the development of doxorubicin-induced senescence and SASP?
- To what extent are senescent cardiac fibroblast, SASP and mitochondrial dysfunction associated with the establishment and progression of myocardial fibrosis and LV dysfunction in-vivo.
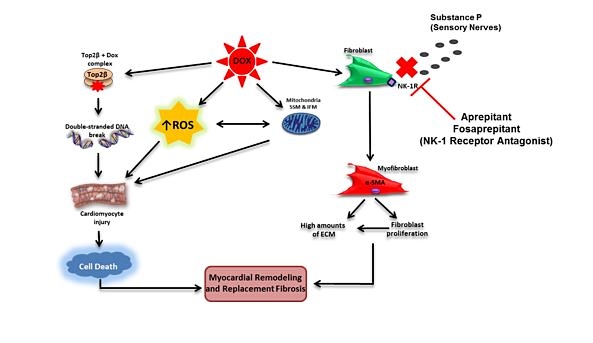
The role of the neurokinin-1 receptor in anthracycline-induced cardiotoxicity
Anthracycline-based chemotherapy remains an essential curative component of adjuvant treatment regimens for triple-negative breast cancer, which constitutes 15-20% of all breast cancers, however, its use is limited by its cardiotoxic effect. This proposal will explore the effectiveness of the neurokinin-1 receptor antagonist to mitigate doxorubicin-induced cardiotoxicity while maintaining doxorubicin’s oncologic efficacy in an established triple-negative cancer model. If the novel cardioprotective effects of these drugs are confirmed without compromising chemotherapeutic efficacy, these drugs could be candidates for rapid clinical repurposing.
Cardiotoxicity in Non-Human Primates
Our lab developed the African Green monkey model of heart failure induced by the chemotherapy medication and we routinely evaluate cardiac function and structure and myocardial tissue using novel cardiac magnetic resonance imaging and mapping techniques to identify the underlying causes of cardiotoxicity. Through our studies, we demonstrated the prognostic value of cardiac imaging biomarkers to forecast the development of LV dysfunction after anthracycline chemotherapy and identify pathophysiologic processes that may be modified to thwart cardiotoxicity.
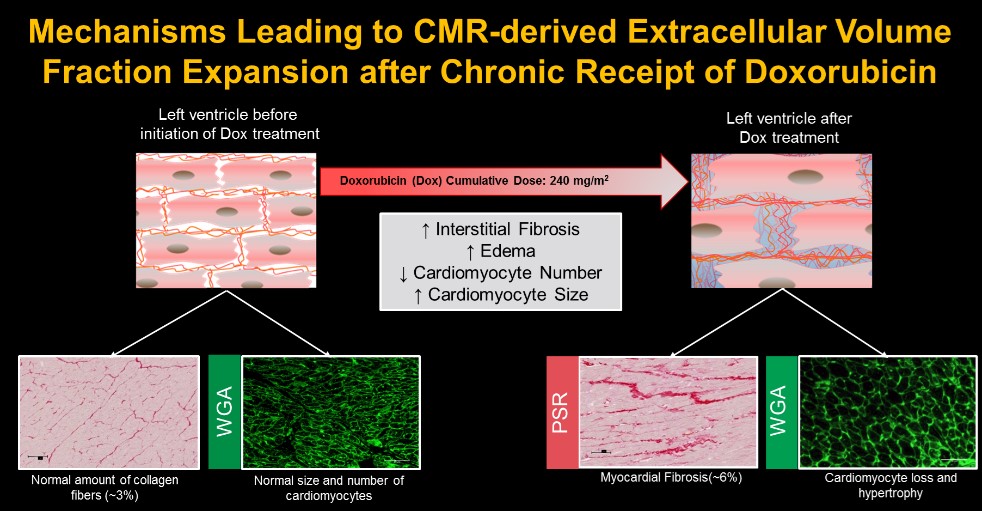
Meléndez GC, et al. Myocardial Extracellular and Cardiomyocyte Volume Expand After Doxorubicin Treatment Similar to Adjuvant Breast Cancer Therapy. JACC Cardiovasc Imaging. 2020 PMID: 31864989
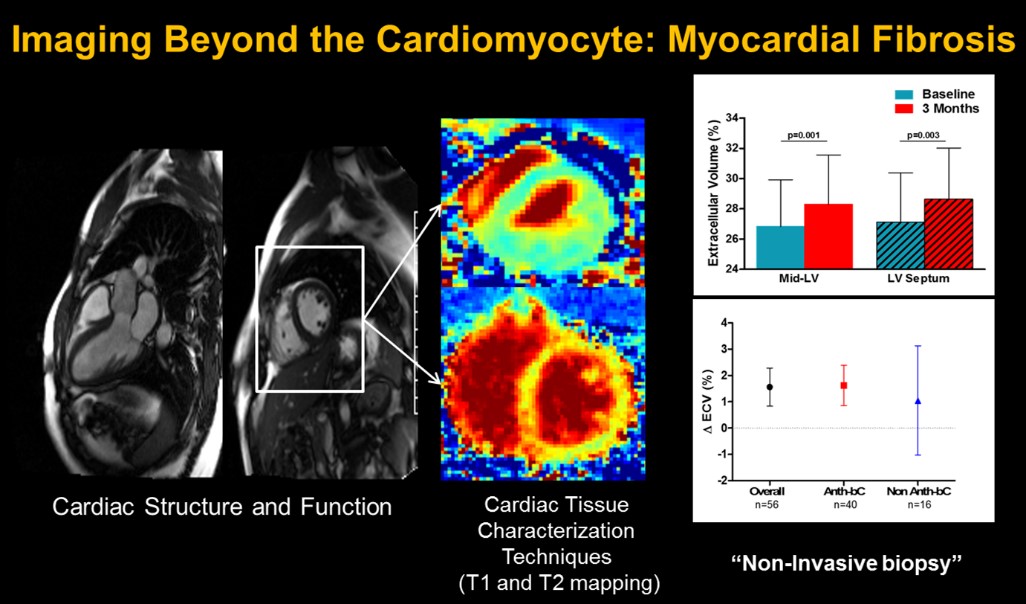
Preventing Anthracycline Cardiovascular Toxicity with Statins (PREVENT)
Our lab is actively participating in this important clinical trial led by Dr. Greg Hundley to evaluate the presence of subclinical myocardial fibrosis and inflammation and their association with the development of left ventricular dysfunction in women receiving adjuvant anthracycline-base chemotherapy for breast cancer. Dr. Meléndez leads the analysis and interpretation of cardiac MRI images, including T1, T2 mapping, and derived extra-cellular volume.
This crucial data will determine whether the early changes after initiation of chemotherapy, forecast the 2-year changes and their association to left ventricular dysfunction. Importantly, after the study is unblinded, we will determine if the administration of atorvastatin was an effective treatment strategy to thwart the subclinical progression of myocardial fibrosis. These data will provide essential information about the prognostic value of the tissue characterization techniques in the cardio-oncology patient.
Collaborative Efforts
Substance P: A Novel Treatment Strategy to Reverse Cardiac Fibrosis Associated to Diabetic Cardiomyopathy
This project is based on a novel hypothesis that Substance P may be an effective treatment strategy for cardiac fibrosis induced by type II diabetes. The overall objective of this proposal is to evaluate the feasibility and safety of exogenous administration of SP as a treatment strategy to reverse cardiac fibrosis and to quantify the changes in cardiac magnetic resonance imaging tissue characteristics (T1, T2, and extracellular volume) and cardiac function before and after treatment in a non-human primate model of diabetes. This project is part of a large research effort lead by Dr. Scott Levick from the University of Sydney and in collaboration with Dr. Kylie Kavanagh at Wake Forest School of Medicine, Comparative Medicine.
Research Team and Trainees
Jessica L. McCanless, MSResearch Laboratory Technician III
Research Laboratory Director
Mentees
Undergraduates
- Hong Kun Lu, 2015
- Maria Paula Ochoa, 2016
- Diego Buitriago, 2016
- Anna Hundley, 2016
- Tancia Bradshaw, 2016
- Adam Burke, 2018
- Ishmael Muhammad, 2020
- Kathleen Kwon, 2020
Master in Biomedical Science Recipient
- Danielle Medina-Hernandez 2017-2019
Medical Students
- Vivian Xu 2017-2020
Internal Medicine and Cardiology Fellows
- Adam Pflum, MD 2016-2018
- Deanna Jones, MD 2016-2017
- Mary Branch, MD 2018-2020
- Carolyn Parks, MD 2018-2020
