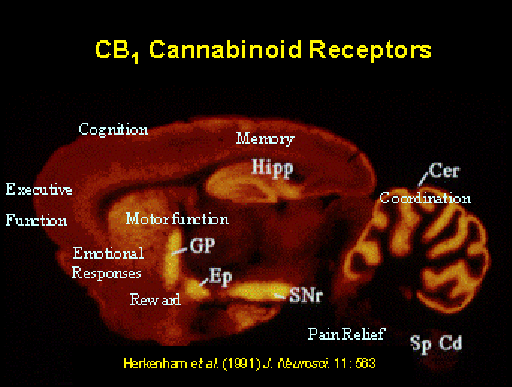
Research at the Howlett Laboratory investigates cannabinoid receptors, the G-protein coupled receptors that respond to a family of eicosanoid ligands in the body (e.g., anandamide, 2-arachidonoylglycerol), as well as to drugs (Δ9-tetrahydrocannabinol, CP-55940 and WIN55212-2). The CB1 cannabinoid receptors are found abundantly in the nervous system and less abundantly in many other organs. The CB2receptors are more readily characterized in the immune system. Evidence exists for other subtypes. We are interested in the mechanisms by which different drugs can activate G-proteins selectively to allow selection of therapeutically relevant signal transduction pathways.
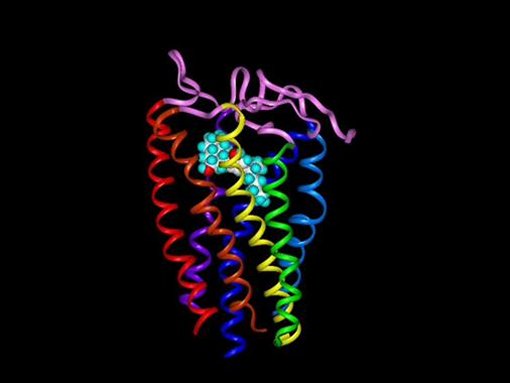
The CB1 Receptor with CP-55244 embedded within its binding site
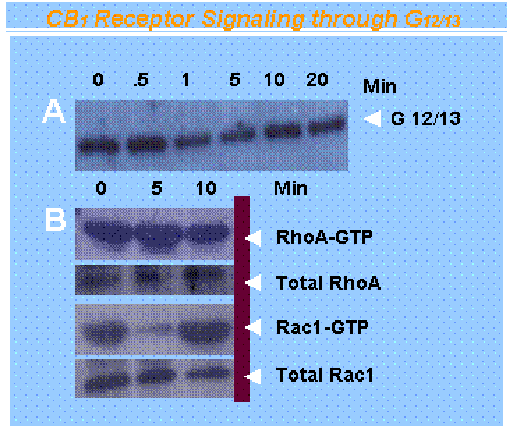
The working hypotheses begin with a model of sequential steps of ligand docking to the inactive receptor-GGDP complex; agonist-induced conformational change in the receptor and concomitant conformational change in the G protein resulting in its activation (GDP/GTP exchange); Ga or Gbg regulation of effectors leading to specific signal transduction pathway activation. Our studies demonstrated that the G-protein selectivity that CB1 intracellular domains prefer. We further demonstrated that agonists of distinct chemical classes influence these different receptor-G protein interactions.
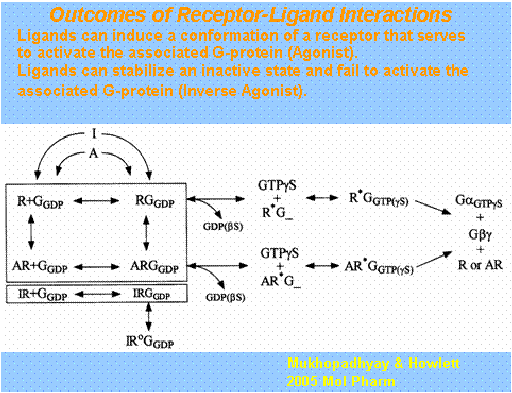
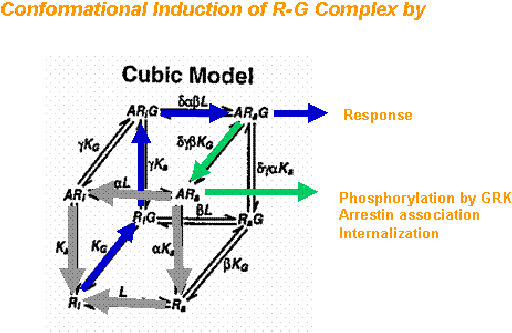
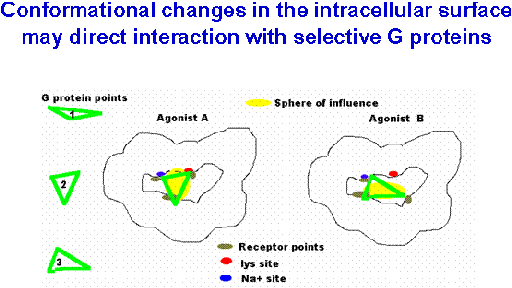
We propose that chemically distinct CB1 ligands can change the conformation of the protein. In addition, phosphorylation can define which signal transduction pathway(s) are activated. We are working to define the types of G-proteins that interact with the CB1 receptor and to investigate the necessary receptor domains and the influence of agonists and phosphorylation.
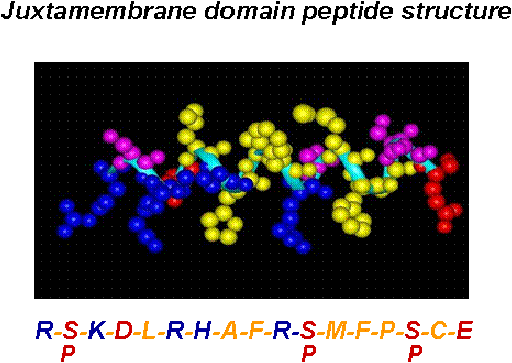
We are investigating the CB1 receptor intracellular surface structure that interfaces with G-proteins. In these studies, we can assess modifications imposed by post-translational events such as phosphorylation. We are also determining the potential for agonist-directed selection of specifici signal transduction pathways. Among those down-stream pathways, we know that adenylyl cyclase inhibition, MAPK activation, Ca2+ regulation, and NO production, are only some of those intracellular events within this diversity of potential signal transduction pathway mechanisms.
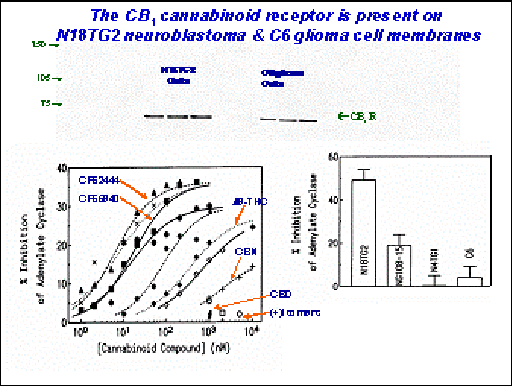
The opportunity for agonist drug design to facilitate selective G-protein-mediated signal transduction pathways may allow stimulation of neuronal cells in brain regions that regulate beneficial effects, such as pain relief and alleviation of spasticity and motor dysfunction of neurodegenerative diseases, while not affecting neurons in brain regions that promote untoward responses such as cognitive dysfunction and memory dysregulation.