Dr. Doug Ririe has been involved in both clinical and basic science research throughout his career. His laboratory research focuses on peripheral neuronal afferent sensory processing. His ultimate goal is to initiate targeted pain treatment regimens and reduce the long-term impact of pain. In particular, novel treatments and interventions are being developed to modulate peripheral nociceptive input responsible for the underlying neuroplasticity in pain syndromes and producing central nervous system effects from pain during critical periods of development.
Research Project Highlights
Chronic pain is a major public health issue affecting 30 perecent of the adult population in the U.S. The burden is immense with lost productivity, utilization of health care resources and a cost over 600 billion dollars per year. Nerve injury induces neural activity that establishes altered processing and long-term behavioral effects. Despite this, incomplete understanding of ongoing chronic pain has resulted in inadequate treatment options.
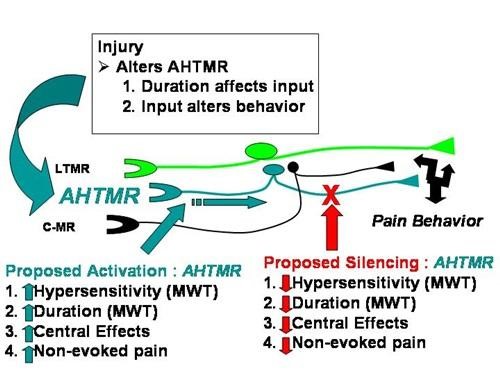
Prior models stress C-fiber drive maintenance of chronic pain and hypersensitivity, but we believe that A-high threshold mechanoreceptor (AHTMR) plays a prominent role in chronic pain. The fast nociceptors, or AHTMR, have long been considered the “first pain” fibers. We show evidence that, long after the “first pain” response is gone, this fiber type does not become an “innocent bystander” in nociceptive/pain processing after nerve injury, but in fact continues and contributes to ongoing nociceptive response.
To investigate this, we are interested in harnessing the power of in vivo intracellular single cell recording and combining this with optically active ion channels to understand the effects of nerve injury on specific subsets of peripheral primary sensory afferents. We also aim to use Designer Receptors Exclusively Activated by a Designer Drug (DREADDs) to either activate or silence AHTMR.
Based on these methods, we hypothesize that:
- AHTMR sensitivity correlates with behavior patterns.
- A duration of AHTMR excitation should impact pain.
- Pain responses will be altered that are sensitive to selective silencing and activating of AHTMRs.
Research Focus
Research Aims
- Establish trajectory of recovery from A-high threshold mechanoreceptor hypersensitivity and correlate with trajectory of behavioral recovery.
- Initial studies demonstrate that AHTMR neurons are hyper excitable after injury with larger receptive fields, lower thresholds and increased stimulus response relationships.
- Establishing trajectory of resolution of both afferent activity and pain behavior might be able to characterize the cellular alterations from injury.
- Hypersensitivity of neurons will be characterized after injury and behavioral response to thermal stimulus are recorded to understand if AHTMR alterations are consistent with the duration of effect on evoked pain behavior after nerve injury and contribute to sustained pain or recovery.
- Characterize afferent activity and selectivity of optically active silencing & excitation.
- We believe based on primary data that active silencing of AHTMRs can be achieved using viral (AAV8) delivery of the optically active proton pumps.
- Our aim is to establish this relationship in vitro and in vivo in single neurons and determine if selective activation of AHTMRS can be achieved with AAV8
- Bi-directional control of AHTMR activity could provide novel tools for exploring their role in pain-related behaviors.
- Measure changes in pain behavior by controlled AHTMR activity.
- Through injection of a biologically inert metabolite of clozapine, clozapine-N-oxide (CNO) as the synthetic ligand to modulate AHTMR activity, we hope to quantify changes of peripheral dendrites of nerve fibers through intrathecal activation.
Select Publications
Templeton TW, Hoke LK, Templeton LB, Ririe DG, Rose DM, Bryan YF. A comparison of 3 ventilation strategies in children younger than 1 year using a Proseal laryngeal mask airway: a randomized controlled trial. J Clin Anesth. 2016 Dec; 35:502-508. PMID: 27871584.
Boada MD, Martin TJ, Ririe DG. Nerve injury induced activation of fast-conducting high threshold mechanoreceptors predicts non-reflexive pain related behavior. Neurosci Lett. 2016 Oct 06; 632:44-9. PMID: 27544012.
Boada MD, Eisenach JC, Ririe DG. Mechanical sensibility of nociceptive and non-nociceptive fast-conducting afferents is modulated by skin temperature. J Neurophysiol. 2016 Jan 01; 115(1):546-53. PMID: 26581873; PMCID: PMC4760509 [Available on 01/01/17].
